The Role of Alloys in Chlorine and Fluorine Production
Exploring the Impact of Alloys in Chlorine and Fluorine Production

In the industrial production of chlorine and fluorine, the role of alloys is pivotal. These elements, known for their reactive nature, require materials that can withstand their corrosive properties during the manufacturing process. Here’s a closer look at how alloys contribute to the safe and efficient production of chlorine and fluorine:
Corrosion Resistance: Alloys, especially those rich in nickel and titanium, are chosen for their ability to resist the corrosive effects of chlorine and fluorine. This resistance is crucial to maintain the integrity of production equipment and ensure safety.
Durability Under High Temperatures: The production of these elements often involves high-temperature processes. Alloys are selected for their ability to maintain strength and stability under such extreme conditions.
Chemical Stability: In environments where chlorine and fluorine are present, alloys provide the necessary chemical stability, preventing unwanted reactions that could compromise the purity of the final product or the equipment used.
Longevity and Cost-Effectiveness: Using alloys in the production equipment extends the lifespan of the machinery, making the process more cost-effective in the long run due to reduced need for replacements and maintenance.
Efficiency in Production: Alloys facilitate a more efficient production process by ensuring minimal downtime due to corrosion or damage and maintaining consistent production rates.
Alloys play an indispensable role in fluorine and chlorine production. Their unique properties, like corrosion resistance, temperature tolerance and chemical stability, make them ideal for handling the challenging conditions of producing these reactive elements.
Chlorine Production

Chlorine has applications in a large variety of areas including:
- Automotive
- Construction
- Defence
- Electronics
- Food Production
- Health Care and Medical
- Metal Production
- Outdoor Recreation and Water Treatment
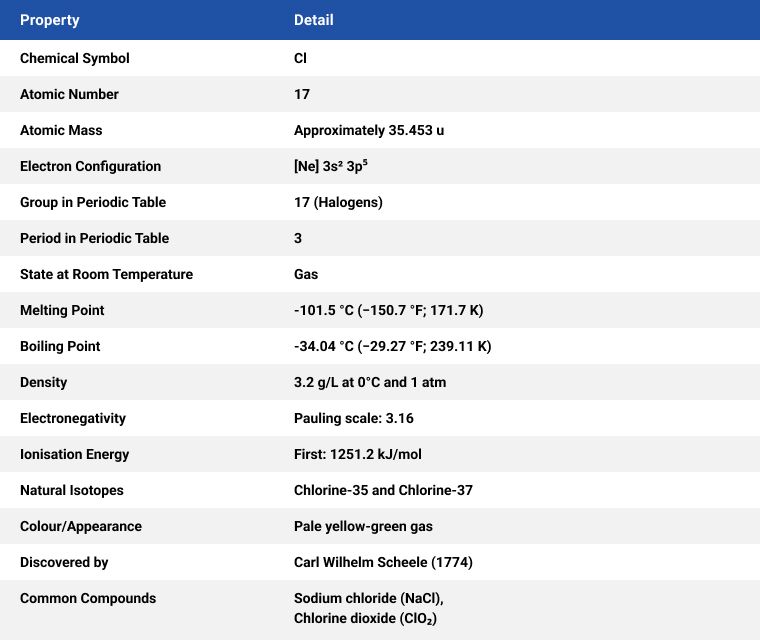
Since its discovery, over 200 years ago, by Swedish researcher Carl Wilhelm Scheele, it has become ubiquitous in the modern world. Chlorine (Cl) is found dissolved in salts in seawater and salt mine deposits. It is a yellowish-green, dense, sharp-smelling gas and appears yellow in liquid form.
Most chlorine is produced through the electrolysis of aqueous sodium chloride and is produced in several ways, the most common method is electrolytically, using the diaphragm, membrane, or mercury cell*1 process, by applying a direct electric current which converts a sodium chloride solution to elemental chlorine. Two additional useful substances are also produced using this process, hydrogen (H) and sodium hydroxide (NaOH).
*1 The mercury cell process is rarely used today due to environmental concerns.
Fluorine (F) Production

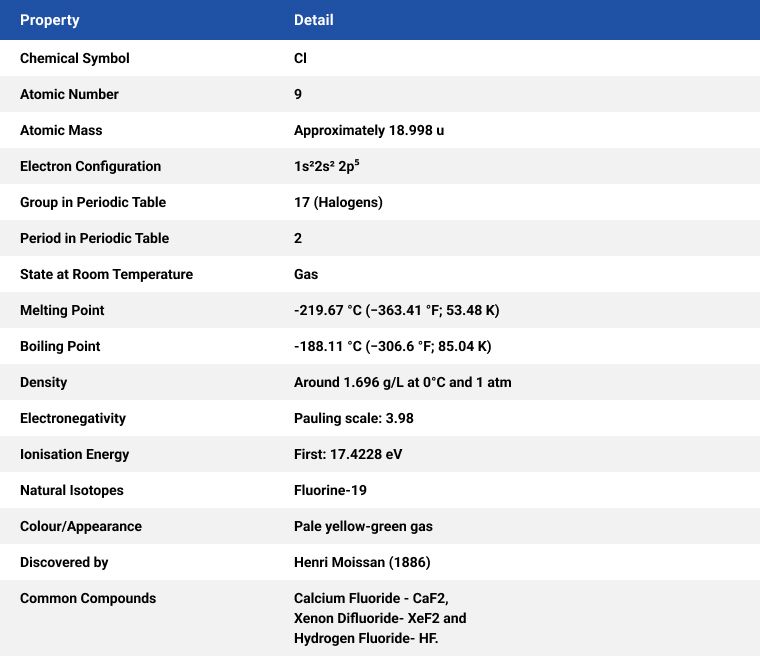
French chemist and pharmacist Henri Moissan won the Nobel Prize in Chemistry (1906) for his work in isolating fluorine from its compounds in 1886.
Fluorine, like chlorine, is a yellowish-green gas with an irritating odour at room temperature. Fluorine is a yellow liquid when cooled. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529, fluorite was added to metal ores to lower their melting points for smelting.
Fluorine (F) is a highly reactive and versatile element with numerous applications across various industries. The greatest use of fluorine is in the nuclear industry to make uranium hexafluoride to produce U-235 isotope and is, therefore, crucial for the nuclear power industry. Some of the primary uses of fluorine are as follows:
- Pharmaceuticals and dentistry
- Agricultural Chemicals
- Teflon Production
- Chemical Industry
- Medical Imaging
- Metallurgy
- Electronics
- Plastics Industry
Fluorine is used to produce fluorocarbons, and their uses include vapour phase soldering of printed circuit boards, leak testing and thermal shock testing of electronics components, refrigerants, dielectric fluids, sterilising agents and lubricants.
Important medical applications also rely on fluorocarbon’s ability to dissolve large volumes of oxygen for organ storage, blood extenders and substitutes for liquid ventilation particularly for premature babies and eye surgery.
Alloys used in the Production of Chlorine and Fluorine
Alloy 22. A nickel-chromium-molybdenum grade alloy, due to its high nickel content is immune to chloride-induced stress corrosion cracking. It demonstrates outstanding resistance to pitting and crevice corrosion particularly in high chloride-containing environments. Alloy 22 has exceptional resistance to a large range of chemical process environments, including ferric chlorides, chlorine, hot contaminated solutions, acetic acids, seawater and brine solutions. Alloy 22 sheet and pipe are frequently specified in chlorine production.
Alloy 200/201. Commercially pure nickel (99.6%) with good mechanical properties and excellent corrosion resistance. By controlling the carbon content, NeoNickel supplies dual-certified Alloy 200/201 which meets the requirements of both alloys.
Alloy 400. A nickel-copper grade alloy, it is one of a limited number of alloys that can be used in conjunction with fluorine and hydrofluoric acid. It exhibits superb corrosion resistance in a wide range of media including fluorine, hydrofluoric acid, sulphuric acid seawater and alkalis. With a high nickel content, this alloy is also immune to chloride-induced stress corrosion cracking.
Alloy 600. A nickel-chromium grade alloy. The high nickel content of Alloy 600 is virtually immune to chloride-induced stress corrosion cracking. The alloy has corrosion resistance to dry chlorine up to 538°C.
Alloy 825. A nickel-iron-chromium grade alloy with additions of molybdenum, copper, and titanium. The nickel content is sufficient for resistance to chloride-ion stress-corrosion cracking.
Alloy C276. A solution-annealed nickel-molybdenum-chromium grade alloy with the addition of tungsten, tungsten inhibits the development of pits. The high nickel content of C276 provides immunity to chloride-induced stress corrosion cracking.
Alloy K500. Is a precipitation-hardened nickel-copper grade alloy. Alloy K500 has outstanding corrosion resistance in a variety of environments and is currently employed in chemical, oil and gas and marine environments.
Of the common commercial metals, nickel-copper alloys such as Monel have been found most versatile in chlorine environments. However, severe corrosion has been encountered under some conditions. Nickel proved to be excellent for service with fluorine to relatively high temperatures.
In a fluorine environment, the chromium-free Alloy 400 is often the material of choice, based on both cost and corrosion resistance. Its major weakness is a propensity to fail by stress corrosion cracking in the event of ingress of air and moisture. Alloy 200 has about the same general corrosion resistance and is immune to SCC. However, the most reliable and cost-effective alloy is Alloy 600 which is the material of choice especially for vaporisers.
Each of these alloys is chosen based on the specific conditions and requirements of the production process, including the concentration of chlorine or fluorine, the presence of other corrosive substances, temperature and pressure conditions. Their use is critical to ensuring the longevity and safety of the production equipment.
Useful links:
Alloy selection for service in chlorine, hydrogen chloride and hydrochloric acid.
FAQs
Why is fluorine highly corrosive?
Fluorine is one of the most corrosive elements due to its high electronegativity and small atomic size. Its electronegativity, the highest of all elements, makes it extremely eager to gain electrons from other substances, leading to aggressive chemical reactions.
This reactivity causes fluorine to readily break bonds in other materials, often resulting in corrosion. Additionally, its small size allows it to penetrate deeply into other materials, exacerbating its corrosive effects. These characteristics make fluorine highly reactive and corrosive, especially in the presence of water, where it forms highly acidic and corrosive hydrofluoric acid.
Is fluorine more toxic than chlorine?
Fluorine is more toxic than chlorine. Both elements are highly reactive halogens, but fluorine’s reactivity is greater, making it more hazardous. This higher reactivity allows fluorine to form harmful compounds more easily, causing severe chemical burns and respiratory issues upon exposure.
Moreover, fluorine’s ability to react with a wide range of substances, including water, makes it particularly dangerous, as it can release other toxic compounds when it reacts. In contrast, chlorine, while still toxic and dangerous, is less reactive than fluorine, making it slightly less hazardous in terms of immediate toxicity.
What makes fluorine the most reactive of all elements?
Fluorine is one of the most reactive elements due to its high reactivity stems from its small atomic size and high electronegativity. Being the most electronegative element, fluorine has a strong tendency to attract electrons from other atoms, leading to the formation of bonds.
This characteristic, combined with its ability to penetrate and weaken molecular structures of other materials, makes fluorine react with almost all elements and compounds, often in a vigorous and sometimes explosive manner. Its high reactivity is why fluorine is handled with extreme caution in chemical processes.
Why is chlorine corrosive?
Chlorine’s corrosiveness is primarily due to its strong oxidising properties. As a halogen, chlorine has a high affinity for electrons, which leads it to readily react with a variety of substances, particularly metals. In these reactions, chlorine tends to strip electrons from other materials, causing oxidation. This process can weaken the chemical bonds of the reacting substance, leading to corrosion.
Additionally, when chlorine is combined with moisture, it can form hydrochloric acid, a highly corrosive substance, further contributing to its corrosive nature. This ability to react with and degrade materials makes chlorine a potent and corrosive chemical, used effectively as a disinfectant but handled cautiously in industrial and chemical environments.
Is fluorine a nonmetal?
Fluorine is indeed classified as a nonmetal. It is part of the halogen group in the periodic table, which consists entirely of nonmetals. Characterised by its high electronegativity and reactivity, fluorine typically forms negative ions by gaining electrons.
This behaviour is a hallmark of nonmetallic elements. Furthermore, fluorine exists as a diatomic gas under standard conditions, another characteristic feature of nonmetals. Its chemical properties, such as its ability to form acidic compounds and its lack of metallic lustre, further reinforce its classification as a nonmetal.
————————————————————————————————————————–

