Why Are Alloys Harder Than Pure Metals

Hardness is the resistance of a material to localised plastic deformation. For example, diamonds are recognised as super-hard, whilst plastic or paper are soft. Alloys are generally harder than pure metals. This is because an alloy is a pure metal with another element(s) added to enhance its properties.
This article will look at the scientific reasons why alloys are harder than pure metals. That includes chemical composition, plus the hardening treatments they can undergo.
What is an Alloy?

An alloy is a pure metal which is combined with other element(s) to enhance its properties. For example, nickel is a pure metal whilst Alloy 600 is an alloy. Alloy 600 is nickel combined with chromium, copper, carbon, manganese, silicon, sulphur and iron.
Understanding Hardness
The scientific definition of hardness is a material’s resistance to localised deformation. This refers to plastic deformation, where the material does not return to its original shape afterwards (unlike elastic deformation). This is normally tested by indentation, abrasion or scratching.
Hardness is different to toughness or strength. Indeed, toughness is the ability of a material to absorb energy before fracture. That means it could dent and still be classed as tough, but not hard.
Strength is the ability of the material to resist deformation by outside pressure. There are two kinds of strength: yield and tensile. Yield strength is the point at which it shows deformation and tensile strength is the point at which it fractures. Strength is different to hardness because it looks at the resistance of the whole material, whereas hardness looks at the surface. A material could scratch and still be strong, but not hard.
Composition Matters: Alloying Elements
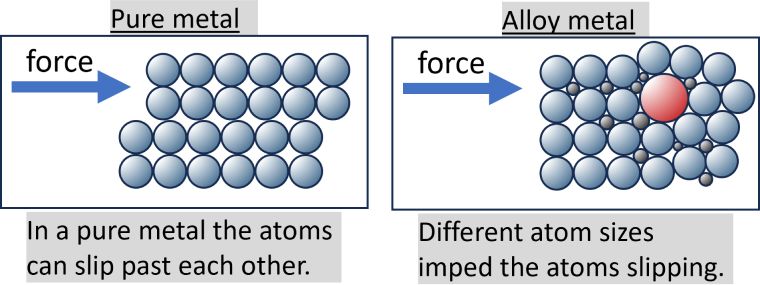
The reason why alloys are harder than pure metals is due to their composition. Alloys are made up of a mix of atoms, which are all different sizes. That makes it harder for the atoms to move over each other, making them resistant to scratching or indentation. Pure metals, on the other hand, have atoms which are all the same size. That means they slide over each other easily and can be prone to scratch or dent.
Solid Solution Strengthening
Solid solution strengthening is a type of alloying process. It involves dissolving one metal into another while both are in liquid strength. One metal acts as the dissolving solution (which will be the base metal) and the other as the solute (the alloying element). There is a solubility limit, which is the maximum amount of solute that can be dissolved. This is why it’s done by trained metallurgists who understand the science behind the process.
Solid solution strengthening makes the alloy stronger and harder than the base metal. This is because the lattice structure is altered, with different sized atoms. That means the atoms are harder to dislocate or move around, so it’s more resistant to deformation.
Precipitation Hardening

Precipitation hardening is a heat treatment process used to increase the strength and hardness of alloys. It keeps the alloy at an elevated temperature for a sustained period of time. This makes certain elements come out of the solution as precipitates. These act as a sort of pin to keep the rest of the atoms in place. This makes the alloy significantly harder and stronger.
Grain Size Refinement
During alloying, as the alloy cools it solidifies and forms grains. If the grains are large, it can leave gaps between them. This makes the alloy weaker and easier to damage. Grain size refinement basically reduces the size of these grains. Smaller grains means smaller gaps between them. This makes the alloy harder, as the grains move less and retain their structure.
Case Study: Alloy Steel

To illustrate why alloys are harder than pure metals, we’ll look at a case study of stainless steel:
Stainless steel is an alloy, with iron as its base metal. Iron is a pure metal, which is known for its ductility and malleability. It also rusts in damp air. Stainless steel, on the other hand, is often used in construction, due to its high strength, hardness and corrosion resistance.
Stainless steel alloy grades all have a slightly different composition. For example, all stainless steels contain a minimum of 10.5% chromium while some grades might also include nickel, molybdenum and manganese. Because it contains a mix of alloying elements, it becomes stronger and harder. This is because it contains lots of different atoms which are all different sizes. This makes it harder to dislocate atoms and scratch or dent it.
Specific alloying elements like chromium contribute to its corrosion resistance. This is because these elements are corrosion resistant themselves.
Alloy Development and Tailoring
There is a lot of science behind the reason alloys are harder than pure metals. Scientists have spent decades studying the composition and structure of materials to learn how to get the best out of them. Metallurgists will refine the balance of elements to produce an alloy that has the desired properties.
We hope this has answered the question ‘why are alloys harder than pure metals’. NeoNickel are a trusted supplier of metal alloys, with a dedicated team of metallurgists on hand to answer any questions. If you would like to order alloys or find out more, please get in touch. Contact us today.
