Corrosion Resisting Alloys for Aqueous Corrosion Applications Across Industries
 Corrosion resisting alloys (CRAs), as the name suggest are alloys that have been designed to and are able to resist degradation in environment or media where they are used. It is worth defining corrosion then as the degradation of a material or alloy as a result of its interaction with its specific environment.
Corrosion resisting alloys (CRAs), as the name suggest are alloys that have been designed to and are able to resist degradation in environment or media where they are used. It is worth defining corrosion then as the degradation of a material or alloy as a result of its interaction with its specific environment.
The Corrosion Phenomenon – Inevitable and Costly.
In the world of materials and alloys, corrosion phenomenon is inevitable and costly.
It is a naturally occurring, thermodynamic change process. As a result, owner/operators need to apply a life cycle cost benefit analysis approach when deciding on material selection investments in their various projects. Unlike other phenomena, corrosion as well as other integrity issues cannot be suspended, deferred and halted in recessionary times; they just simply go on as they are no respecter of economic cycles.
The good news is that corrosion can be controlled and slowed down dramatically; say to rates less than 0.1mm/year. This is achieved by right materials selection, design, coatings inhibition and cathodic protection as applicable in various industries.
 How do Corrosion Resistant Alloys protect against Corrosion?
How do Corrosion Resistant Alloys protect against Corrosion?
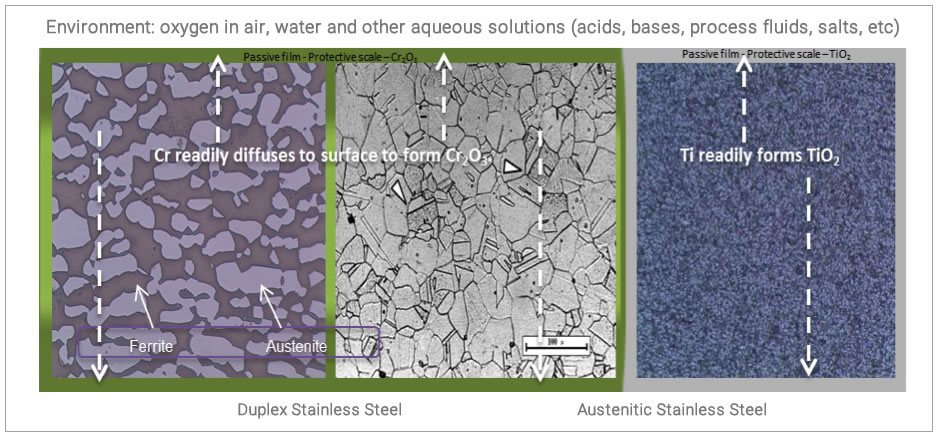
CRAs rely on the existence and stability of a passive film on its surface to act as a protective barrier to deleterious interactions between the environment and component.
The passive film is usually chromium oxide for most stainless steels, nickel alloys, cobalt based alloys and titanium oxide in titanium alloys. This passive film is extremely thin – in order of nanometres (1 – 5 nm), very adherent and invisible to the naked eye. They readily rapidly form and are very stable in the presence of oxygen in air, water and other aqueous solutions (acids, bases, process fluids, salts, etc.). The presence of chromium, alongside other alloying elements such as molybdenum, nitrogen, tungsten and copper in CRAs does improve corrosion resistance of alloys in diverse environment.
Due to this passive film presence, general uniform corrosion is seldom an issue, what is more prevalent is localised corrosion inform of pitting corrosion, crevice corrosion and stress corrosion cracking, leading to other failure mechanisms such as galvanic corrosion and microbial induced corrosion. This will thus manifest initially as a pit or crevice, then crack, fracture and eventual collapse.
The key drivers or influencers of this type of corrosion are oxygen, halides (especially chlorides), temperature, and flowrate, presence of impurities or contamination and microbial activity from organic matter.
How are CRA’s Ranked?
CRAs are ranked for performance in process, chemical, oil and gas industry by a unique number called the Pitting resistance equivalent number PREn, which takes into account the effect of specific elements such as chromium, molybdenum, nitrogen, and sometimes tungsten; as they contribute to localised corrosion resistance of individual alloys.
The number is calculated based on the formulae;
PREn = %Cr + 3.3 (%Mo) + 30 (%N) or PREw = %Cr + 3.3 (%Mo + 0.5%W) + 30 (%N)
Some of the most common CRAs with description are detailed in the table below;
| Corrosion resistant Alloys | Nominal Chemical Composition | Description | ||||||
| \*PREn | Alloy | Ni | Cr | Mo | Fe | Others | ||
| 48 | AL-6XN® | 24 | 20.5 | 6.3 | 48 | C: 0.02, | A high (6.3%) molybdenum super austenitic stainless steel, with high strength. Superior resistance to chloride pitting and crevice corrosion. | |
| N08367 | N: 0.22 | |||||||
| 27 | Alloy 20 | 33 | 19.5 | 2.2 | 40 | C: 0.02, | An austenitic stainless steel for sulphuric acid corrosion environments. Resists intergranular corrosion as welded. Resistant to chloride and polythionic acid stress corrosion cracking. | |
| N08020 | Cb + Ta: 0.5, | |||||||
| Cu: 3.3. | ||||||||
| 29 | LDX 2101® | 1.5 | 21.5 | 0.3 | 70 | Mn: 5.0, | A lean duplex stainless steel resistant to stress corrosion cracking. Comparable to 316L in general corrosion, economical and high strength. | |
| S32101 | N: 0.22, | |||||||
| C: 0.03 | ||||||||
| 28 | 2205 | 5.6 | 22.1 | 3.1 | 67 | C: 0.02, | Duplex austenitic – ferritic stainless with high resistance to chloride stress corrosion cracking and to general corrosion. High strength. | |
| S32205/S31803 | N: 0.16 | |||||||
| 43 | ZERON® 100 | 7 | 25 | 3.5 | 62 | C: 0.02, | Super duplex stainless steel with high strength and high resistance to chloride pitting corrosion and sulphuric acid. Suitable for seawater service with a minimum PREn 40. | |
| S32760 | Cu: 0.7, | |||||||
| W: 0.7, | ||||||||
| N: 0.22 | ||||||||
| 51 | 625 | 61 | 21.5 | 9 | 4 | Cb: 3.6 | A high strength (9%) molybdenum nickel alloy with excellent resistance to hot seawater, scrubber environments and reducing acids. | |
| N06625 | ||||||||
| 29 | 718 NACE/API | 52 | 19 | 3 | 19 | Al: 0.5, | 718 is a precipitation hardened nickel – chromium alloy. It combines high strength in the aged condition with good corrosion resistance and weldability. Commonly used in oil and gas exploration. | |
| N07718 | Ti: 0.9, | |||||||
| Cb+Ta: 5.0 | ||||||||
| Alloy 400 | 63 | – | – | 2.5 | Cu: Rem, | Alloy 400 from NeoNickel is used for its superb combination of corrosion resistance, weldability and ductility. The corrosion resistance in seawater is especially good under high velocity conditions. | ||
| N04400 | C: 0.30, | |||||||
| Mn: 2.00, | ||||||||
| Si: 0.50 | ||||||||
| 65 | C 276 | Rem | 15 | 15 | 7 max | C: 0.02 max | C276 is a solid-solution strengthened, nickel-molybdenum-chromium alloy with a small amount of tungsten, which exhibits excellent corrosion resistance in an assortment of harsh environments. | |
| N10276 | Mn: 1 max | |||||||
| W: 4.5 max | ||||||||
| 65 | C 22 | 52 | 22 | 13 | 6 | C: 0.015 max, | Alloy 22 is a fully austenitic, nickel-chromium-molybdenum-tungsten alloy with high corrosion resistance compared to other nickel-chromium-molybdenum alloys. The high chromium content provides good resistance to oxidizing conditions while the molybdenum and tungsten content give good resistance to reducing media. This alloy provides exceptional resistance to pitting, crevice corrosion, general corrosion and stress corrosion cracking. | |
| N06022 | Co: 2.5 max, | |||||||
| Mn: 0.5 max, | ||||||||
| W: 3.5 max | ||||||||
| Titanium alloys | Ti | O2 | C | Fe | Others | |||
| Grade 2 Unalloyed R50400 | Rem | 0.25 max | 0.10 max | 0.3 | H: 0.015 max | Titanium CP3 Grade 2 possesses excellent welding properties, excellent resistance to oxidation and corrosion, good strength and superb cold forming properties making it the alloy of choice for industries globally. | ||
| max | N: 0.03 max | |||||||
| Grade 5 | 89.4 | 0.16 | 0.10 max | 0.15 | Al: 6.0, | Ti 6Al-4V (Grade 5) is the most widely used of all the alpha-beta titanium alloys. It is typically used in the annealed condition, at service temperatures through 399°C. It offers high strength and excellent general corrosion resistance. | ||
| Ti 6Al-4V | V: 4.0 | |||||||
| R56400 | ||||||||
| \*PREn is calculated based on actual ladle analysis chemistry thus may vary | Al 6XN®, LDX 2101®, ZERON 100® are registered trademarks of Allegheny Ludlum, Outokumpu Stainless and Rolled Alloys respectively. | |||||||
 Common Aqueous Environments and Suggested Alloys
Common Aqueous Environments and Suggested Alloys
Corrosion in component parts, equipment or assets can be expensive, especially in difficult to reach environment or high shutdown cost application.
More unforgiving is localised corrosion as it occurs very fast and the damage is usually done, then a realisation. This suggests a real need for expert advice to minimize risk, damage and impact to environment, costs and above all health and safety concerns.
The table below is a performance guidance showing relative resistance of some of the most common aqueous application environment;
| Wet corrosion | Environment | Not suggested | Good | Better | Best |
| Performance guide | Chlorides | 304L. | Alloy 20, 316L, | 400(a), 2205, 317L. | AL-6XN, 625, C-276, |
| (Pitting, crevice corrosion) | LDX 2101, 600. | Titanium, C22, | |||
| Alloy 59, ZERON 100. | |||||
| Chloride stress corrosion cracking | 304L, 316L. | LDX 2101, 904L, 2205, | AL-6XN, Alloy 20, | 400, 600, 625, | |
| 317L | ZERON 100. | Alloy 59, C-276, C22. | |||
| Hydrochloric acid | Titanium(b), 600, Alloy 20, 2205, | 200(a), 400(a),625, | C22, C-276, 686. | Zirconium(a), | |
| LDX 2101, 317L. | ZERON 100 | HASTELLOY® B2, B3 (a), | |||
| Tantalum, Titanium (b). | |||||
| Hydrofluoric acid | 200, 600, 2205, etc. | C-276, C22, | 400(a), Silver(a) | Gold, Platinum. | |
| Alloy 59, | |||||
| 400 (N purged). | |||||
| Sulphuric acid | Titanium, 600. | 316L, 317L, LDX2101,2205 | AL-6XN, 625 | Alloy 20, C-276, Tantalum, | |
| ZERON 100. | |||||
| Phosphoric acid | 200, 400, 316L, 317L. | 904L, 2205 | AL-6XN, Alloy 20, | G-30, 625. | |
| (commercial) | ZERON 100. | ||||
| Nitric acid | 904L, AL-6XN, 200, | 304L, Alloy 20, 2205, ZERON 100 | 625 | Zirconium, Tantalum. | |
| 400, 600. | |||||
| Caustic | 304L, 316L, 317L, | Alloy 20, 2205, | 600, 625, 400, 686, C22, C-276. | 200(a). | |
| Tantalum. | LDX2101, | ||||
| ZERON 100. | |||||
| (a)Presence of oxygen or oxidizing salts may greatly increase corrosion. (b)Titanium has excellent resistance to hydrochloric acid containing oxidizers such asFeCl3, HNO3, etc. However, titanium has very poor resistance to pure, reducing, HCl. This chart is intended as guidance for what alloys might be tested in a given environment. It must NOT be used as the major basis for alloy selection, or as a substitute for competent corrosion engineering work. | |||||
| Hastelloy® is a registered trademark of Haynes International | |||||
You could contact NeoNickel for support and as your CRA vendor in Europe.
Our metallurgist and corrosion engineers have an in-depth knowledge and vast experience across industries in corrosion applications and are happy to support clients regarding advice with material related issues, alloy performance and material failure analysis.