Introduction to the Structure and Properties of Metal Alloys
Metals account for about two thirds of all the elements on Earth and about 24% of the mass of the planet.
All elements, including metals are composed of atoms. All atoms in the element are identical.
The number of protons in an atom is called its atomic number and defines the element.
The periodic table arranges all the elements in the universe by their atomic number. For instance, carbon has six protons while iron contains 26. It is these differences in the number of protons and neutrons in atoms which account for all the different elements.
Alloys are a mixture of two or more elements where one element is a metal. These elements can combine in a chemical process called metallic bonding.
A metallic bond is a type of strong chemical bond which occurs in pure metals and alloys only.
Metallic Bonding
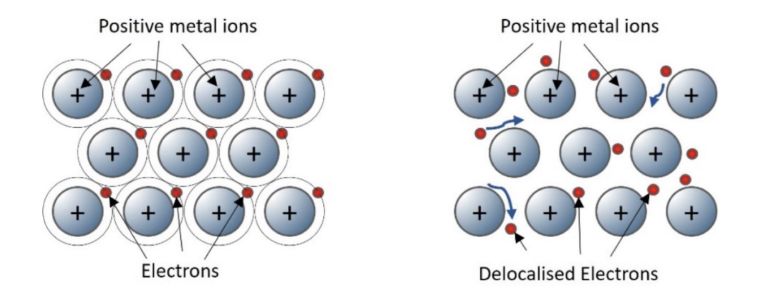
Pure metals have three-dimensional giant structures in which positive metal ions are arranged in layers surrounded by electrons.
Metallic bonds are created when the outer shell electrons become delocalised and surround the positive ions creating a strong electrostatic force of attraction.
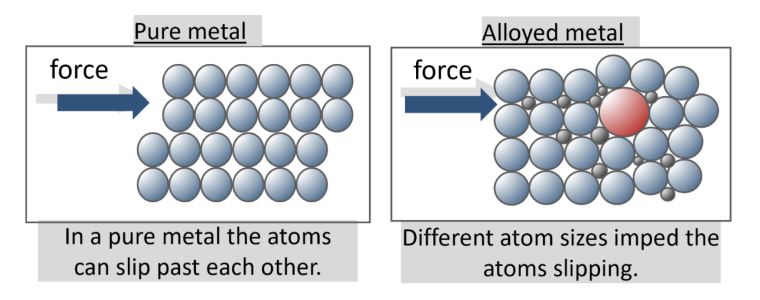
The atoms in a metal are closely packed and arranged in a regular pattern. They are held together by the strong metallic bond but still enable atoms to move; otherwise, how could metals be hammered into sheets or drawn into wires?
Alloys are generally harder than the pure elements they contain, this is due to the pure metal atoms being the same and arranged in layers, as opposed to alloys which contain elements with atoms of different sizes.
When a metal or alloy is processed, the atoms within each grain are lined up in a specific pattern, depending on the crystal structure of the sample. The grain size influences the mechanical property of a material, especially the strength. There is a much greater chance for a dislocation to be stopped at a grain boundary in the smaller grain, therefore the smaller grain is stronger.
Substitutional Alloy
When the atoms of different elements are relatively similar in size, an exchange occurs when the atoms of the metallic crystals are substituted with atoms of the other constituents.
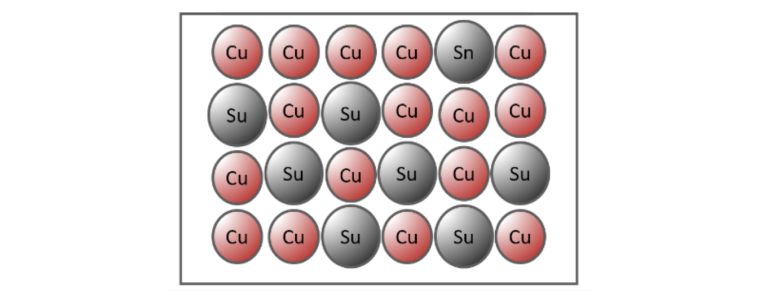
Bronze is an example of a substitutional alloy where copper is substituted for tin.
Interstitial Alloy
When non-metal atoms are sufficiently small, they are accommodated within the metal lattice without, or to only a small degree, any distortion of the metal lattice.
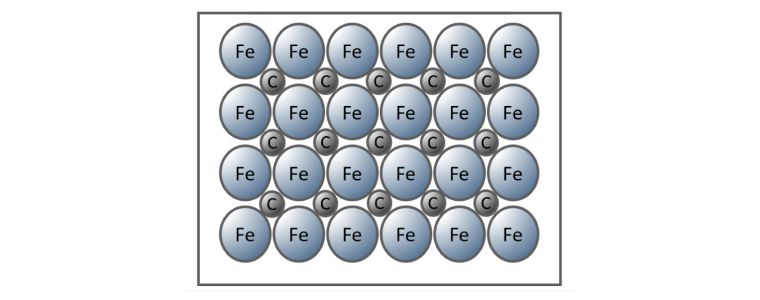
Steel is an example of an interstitial alloy where the carbon atoms fit between the iron atoms.
Combined Substitutional and Interstitial Alloy.
The third option is a combination of the previous two, some atoms are exchanged and others are accommodated between the metal lattice.
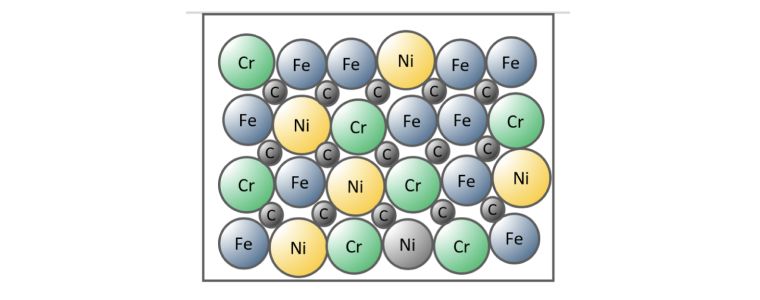
Stainless steel is an example of a combined substitutional and interstitial alloy, where iron is substituted for chromium and nickel and the carbon atoms fit between the metal atoms.
Crystal Structures
Metal atoms are packed together as closely as possible to form the strong metallic bonds with several ‘packing arrangements’ possible. To understand crystal structures lets substitute atoms with footballs. Imagine I need to pack footballs in a box,
I create layer 1, packing the footballs on the bottom of the box in neat orderly rows. Layer 2 of the footballs, fit into the troughs made by the first layer.
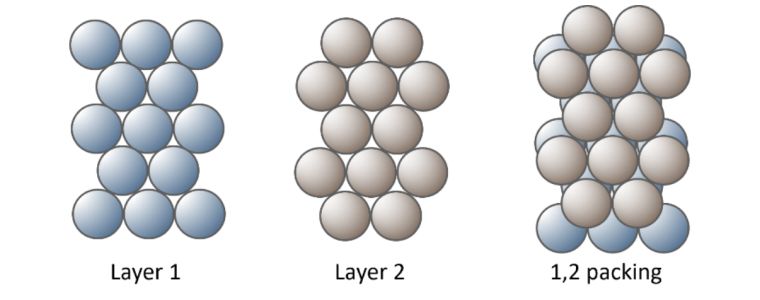
Continuing this packing arrangement with alternative layers could be designated as 1, 2. 1, 2. 1, 2.
This is referred to as Hexagonal Close Packed.
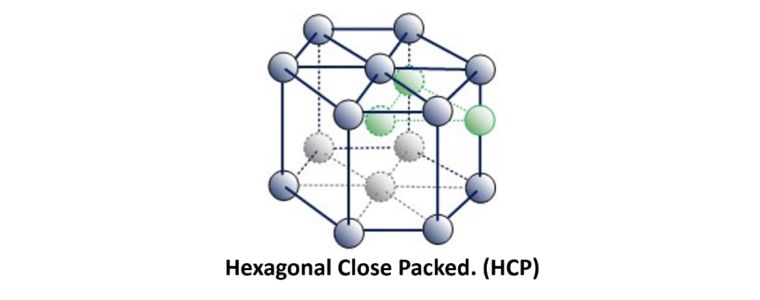
Examples of HCP metals include Beryllium, Cadmium, Magnesium, Titanium, Zinc and Zirconium.
If layer 3 of the footballs are packed so that they do not lie over either layer 1 or layer 2, this could be designated as 1, 2, 3. 1, 2, 3. 1, 2, 3.
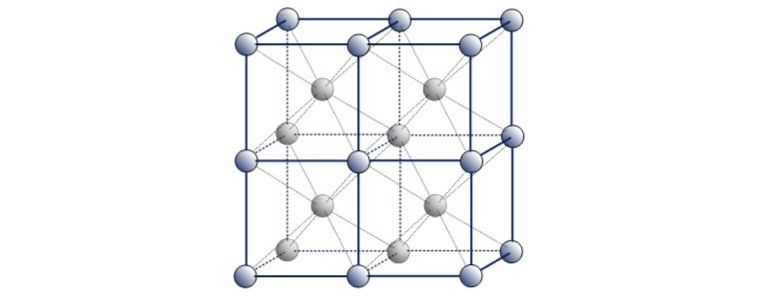
This is referred to as Face Centered Cubic.
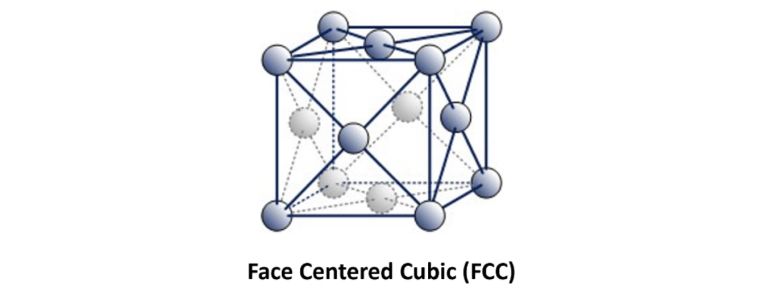
Examples of ‘Austenitic’ FCC metals include Aluminium, Copper, Nickel, Gamma Iron, Gold and Silver. Austenitic steel is a type of stainless steel that contains at least 10.5% chromium and 8% nickel.
A third common packing arrangement in metals is the Body-Centered Cubic (BCC) unit cell which has atoms at each of the eight corners of a cube plus one atom in the centre of the cube.
Because each of the corner atoms is the corner of another cube, the corner atoms in each unit cell will be shared among eight-unit cells. The BCC unit cell consists of a net total of two atoms, the one in the centre and eight eighths from each of the corners.
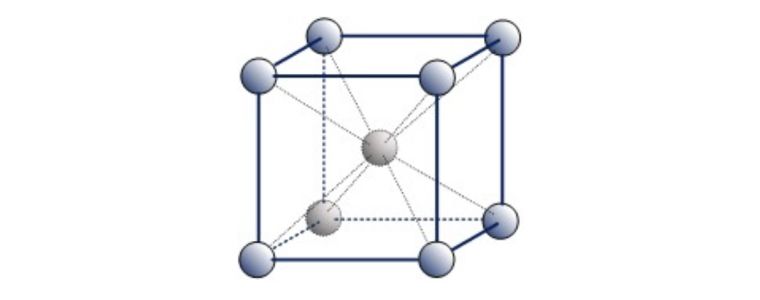
Body Centered Cubic (BCC)
Examples of ‘Ferritic’ BCC metals include Alpha Iron, Tungsten, Chromium & Beta Titanium. Ferritic steel is a type of stainless steel that contains less than 10% chromium.
BCC Lattice
Duplex alloys contain a combination of BCC and FCC structures.
Alloying elements can be added to promote FCC (for example nickel) or BCC (for example chromium).
It is the variety and proportion of each element combined with the manufacturing process and treatment which give alloys their specific properties.
Alloy Properties
To one degree or another metal alloys display the following properties:
Strength – Tensile. Tensile strength refers to the amount of load a section of metal can withstand before it breaks.
Yield Strength. – The yield strength or yield point of an alloy is defined as the stress at which a material begins to deform plastically. Prior to the yield point the material will deform elastically and will return to its original shape when the applied stress is removed.
Hardness. – hardness is a characteristic that determines the surface wear and abrasive resistance. The ability of a material to resist denting from impact is related to its hardness as well as a material’s ductility.
Ductility. – ductility is a metal’s ability to being drawn or stretched without breaking. It is one of the crowning mechanical properties of a metal. Without a sound understanding of the science of ductility, manufacturers would be unable to guarantee the safety of their machinery.
Malleability. – malleability can be confused with ductility, but metal can be malleable without being ductile. While ductility refers to the capacity of metal to stretch (tensile stress), malleability refers to its capability to shape (compressive stress). Shaping could mean being pressed or rolled into thin sheets for example.
High melting point – High temperature alloys. – Metal alloys have a higher melting point than their constituent metals. The reason behind this behaviour is the presence of additional elements in the alloy, which causes changes in the crystal structure of the metal. A high temperature alloy broadly refers to those alloys which provide strength, environmental resistance and stability within the 260°C – 1205°C temperature range.
Thermal conductivity. – thermal conductivity is a measure of an alloys potential to conduct heat.
Electrical conductivity. – is an alloys’ ability to enable the flow of electric current through them. Alloys have lower electrical conductivity than pure metals. The electrical conductivity of a metal is enabled by the delocalised electrons discussed earlier. As a material erodes, it becomes less conductive.
Resistance to corrosion. – Corrosion resistance is the capacity to maintain the binding energy of a metal and withstand the deterioration and chemical breakdown that would otherwise occur when the material is exposed in a corrosive environment. (All environments are eventually corrosive).
Density. – density refers to how heavy an alloy is, the weight is determined by how many atoms are packed into the material.
Grain Size. – grain size and geometry have a significant impact on the physical and mechanical properties of an alloy, such as hardness, yield strength, tensile strength. The grain size requirements are specified in the ASTM standard for certain grades to ensure correct manufacturing controls are in place.