The Properties of Metals and Alloys & How They Affect Use

Classifying and identifying metals and alloys is a crucial skill for anyone that intends to buy them for commercial purposes.
If you’re more familiar with the types of alloy that’ll help you solve your problem or achieve a goal, the consultative period is quicker and simpler.
This guide will provide information about the properties of metals and alloys, which will be based on factors like ductility, load capacity, elasticity and strength.
Testing these factors allows us to build and extend our range of alloy metal parts, and adapt them, so they’re suitable for all types of projects from aeroplane building to chemical processing.
By knowing the properties of various metals, you’ll be able to determine what metal suits your project and make a more informed decision. We’ll include examples, so you have a point of reference throughout the article.
What Are the Properties of Metals and Alloys?
The development of alloys that perform specific functions within complex machines or production processes is reliant on the particular properties of that alloy.
We group properties into various categories, such as optical properties, manufacturing properties and environmental properties, but we’re going to focus on the physical, chemical and mechanical properties.
- Physical Properties – physical properties relate to the characteristics of a metal or alloy that can be measured without any change in circumstance, such as density.
- Chemical Properties – chemical properties relate to the characteristics of a metal or alloy that become evident upon experiencing a chemical reaction, such as flammability.
- Mechanical Properties – a mechanical property that relates to the performance of the metal or alloy under conditions like heat or pressure, such as ductility.
Properties of Metals & Alloys
There are more than 100 properties a material could have, but we’re going to focus on the ones we think are most important for metals and alloys, they are:
Mechanical Properties
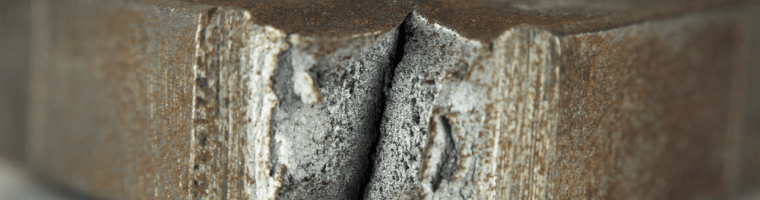
Brittleness
Metal is brittle if it breaks when it’s exposed to small amounts of stress. Often, brittle material will create a ‘snap’ sound when it breaks, such as plastic or ceramics. The alloy steel is brittle at low temperatures, but this depends on composition and processing.
Cast iron is known for being a brittle metal. It has a low tensile strength which means it will break before it bends. Because it cannot be bent without breaking, casts are used to shape the metal in its liquid form.
One of the most popular uses of cast iron is skillets, pans and pipes; the automotive industry also uses cast iron for cylinder heads, cylinder boxes and gearbox heads.
Ductility
Ductility is metal’s ability to be pulled away or elongated into a wire without breaking. Think about taking a lump of plasticine in your hand and slowly pulling it in opposite directions with either hand. You can see the wires in the plasticine start to break as you pull it apart. It’s the same process with metal, but it isn’t as obvious.
There are plenty of ductile metals, such as copper and platinum. Copper is commonly used for wiring and platinum is stretched out to make jewellery. Platinum is the most ductile metal.
Malleability
People often get malleability confused with ductility, but metal can be malleable without being ductile.
While ductility refers to the capacity of metal to stretch (tensile stress), malleability refers to its capability to shape (compressive stress). Shaping could mean being pressed or rolled into thin sheets. For example, you could take a lump of gold or aluminium and press it into a thin sheet to make gold leaves.
However, this is a rare use of malleable metal, and you can find them in most areas of life, such as aluminium tin cans, ornaments and kitchen utensils.
Elasticity
Elasticity refers to the capacity of metal to be deformed and then move back into its original shape. Contrast this to ductility and malleability where the metal stays in the position it is stretched or shaped into.
For example, let’s consider steel wire and rubber wire. Which one do you think is more elastic? It seems like a rubber wire would be the logical answer, but it’s wrong. Rubber is more stretchable than steel, but it doesn’t go back to its original formation after the stress is reduced, whereas steel does. The wire would snap after a certain amount of pressure, whereas steel would move a little and move back a little.
Most metals are elastic to some degree, as the range of movement will be small.
Hardness
Hardness refers to the ability of metal to resist denting or abrasions following force or impact. Because there are so many different methods of testing hardness, the correct definition is often confused. For example, hardness has nothing to do with ductility or brittleness; metal can be hard, as well as ductile or brittle.
Tungsten is one of the hardest alloys on the planet. It is often used in mining to create drilling and excavation tools, as well as marine vehicles, aircraft turbines and blades in stationary power supplies.
Fatigue
Fatigue is the weakening of metal after a period of pressure is applied, causing localised structure damage and cracks in the metal. Once a crack has appeared, the sequence of pressure will continue to worsen it until it gets to a critical size and the metal fails.
Most metals will experience some fatigue, but it isn’t easy to tell on some of them. For example, aluminium is rumoured to have no fatigue limit, which isn’t true. The fatigue limit on aluminium isn’t as apparent as the fatigue limit on steel, so it’s a challenge to know when it will fail.
Physical Properties

Density
When we talk about density, we’re talking about how heavy metal is. The weight of metal is determined by how many atoms are packed into the surface area. Density refers to how the metal interacts with other materials.
For example, the densest metals osmium and iridium would sink in water because it is denser, whereas lithium would float due to its low density.
Dense metals have plenty of applications such as bullets and radiation shielding (lead), and weights from anchors to paperweights – which will often be covered in a ‘prettier’ chrome.
Melting Point
The melting point of the metal is quite simple; it’s at what point the metal changes from a solid to a liquid. At this point, the metal is in equilibrium between both states.
The melting point is important because most metals and alloys are combined at the liquid point. For example, to make an alloy wheel, you would liquify aluminium and magnesium, and add other elements.
The melting point of metal will determine what is it used for, for example, tungsten has one of the highest melting points, so it is used in lightbulbs to combat the heat generated.
Chemical Properties

Corrosion Resistance
Corrosion is the gradual destruction of metal by chemical or electrochemical interaction with the environment, which is usually oxygen or sulfates.
Rusting is a typical example of corrosion, which is the formation of iron oxides on the metal. There are numerous ways to protect from corrosion, such as surface treatments or cathodic protection.
Stainless steel is a prime example of a corrosion-resistant metal. It’s strong, easy to maintain, has a long life cycle and is recyclable. Stainless steel is used in countless day-to-day products such as zips, fridges and huge structures like The Chrysler Building and the One World Trade Center.
Reactivity
Reactivity refers to how metal interacts with part of its surrounding environment, like air or water. Some metals are more reactive than others and will react vigorously to an encounter with air or water.
Potassium is the most reactive metal when it comes into contact with water and air. The oxygen in the air causes the potassium to burst into a purple flame, and it tarnishes at room temperature. When placed into water, potassium bounces around on the top of the water with the same purple flame and dissolves into a colourless liquid.
Just because metal is reactive, it doesn’t mean it can’t be used in the alloying process; for example, magnesium is often combined with other metals to prevent rust.
What is An Alloy Metal?
If you’re confident you know what the properties of metals and alloys are, you should move on to our next article, where you’ll discover the basics of alloy metals and why they’re useful.
